Román R.a, Rodón N.a, Verdú M.a,c, Calvo M.d, García-Peláez B.a, Díaz O.a, Biern G.a, Serrano T.c, Puig X.a,b,c
a BIOPAT, Biopatologia Molecular SL, Grup Assistència, Barcelona, Spain. b Hospital de Barcelona-SCIAS, Grup Assistència, Barcelona. Spain. c Histopat Laboratoris, Barcelona, Spain. d Statistics Department, Universitat de Barcelona, Barcelona, Spain.
Resumen
El estudio de la inestabilidad de microsatélites (MSI) es altamente recomendable en todos los carcinomas colorrectales, tanto como una primera aproximación para identificar posibles pacientes con síndrome de Lynch, como debido a su valor pronóstico y a su asociación con una mala respuesta a los regímenes basados en 5-fluorouracilo. A pesar de estas evidencias, la aplicación del estudio de MSI en la práctica general es bastante limitada. Este hecho pone de relieve la necesidad de herramientas de cribado para facilitar la implementación del estudio de MSI. Este trabajo presenta la validación de dos modelos de predicción previamente publicados, RERtest6 y RERtest8, basados en parámetros clínico-patológicos y dirigidos a una población no seleccionada por edad. La serie incluye 206 tumores colorrectales primarios de 199 pacientes, a los que se les aplicaron los modelos de predicción que contienen 6 ó 8 parámetros, respectivamente, antes de la evaluación del estado de MSI con el panel NCI consenso o el kit de MSI Promega. Se detectó alta inestabilidad de microsatélites (MSI-H) en 21 casos (10,1%). Ambos modelos han confirmado su robustez y fueron capaces de mantener valores predictivos negativos cercanos al 95%, lo que permite la reducción del número de casos que requieren un estudio molecular al 10%. Asimismo, la naturaleza de los parámetros incluidos en los modelos, que en su mayoría ya forman parte de un examen histopatológico de rutina, los convierten en una herramienta útil y de fácil aplicación en la práctica clínica.
Palabras clave: Inestabilidad de microsatélites, modelo de predicción, cáncer colorrectal, parámetros histopatológicos, síndrome de Lynch.
Abstract
Determining microsatellite instability status (MSI) is now highly recommended in all diagnosed colorectal carcinomas, both as a first approach to identify putative Lynch syndrome patients, and due to its strong prognostic value and association with a poor response to 5-fluorouracil-based regimes. Despite such evidences, the implementation of MSI testing to general practice is quite limited. This fact highlights the need for screening tools to make MSI testing easier to implement. This study presents the validation of two previously published prediction models, RERtest6 and RERtest8, based on clinicopathological features and aimed at a non-aged restricted population. The series includes 206 primary colorectal tumors from 199 patients, to which the models containing 6 or 8 parameters were applied before the assessment of MSI status using the consensus NCI panel or the MSI Promega kit. High-level microsatellite instability (MSI-H) was detected in 21 cases (10.1%). Both models confirmed their robustness and were able to maintain negative predictive values close to 95% which allow the reduction of cases to be tested by molecular methods to 10%. Furthermore the nature of the parameters included in the models, mostly already part of a routine histopathological examination, makes them a useful and easily implemented tool for the routine practice.
Keywords: Microsatellite instability, prediction model, colorectal cancer, pathological parameters, Lynch syndrome.
INTRODUCTION
Approximately 10-15% of colorectal carcinomas (CRC) exhibit high-level microsatellite instability (MSI-H), characterized by a hypermutated phenotype and caused by the loss of DNA mismatch repair (MMR) activity. This MMR deficiency can either be due to the presence of germ-line mutations in the MMR genes, MLH1, MSH2, MSH6 or PMS2, which give rise to hereditary nonpolyposis colorectal cancers (HNPCC) or Lynch syndrome, or due to epigenetic silencing of the MMR gene MLH1 by promoter methylation in sporadic cases.
Identifying the 2 to 3% of hereditary cases has an obvious impact on both the patients and their relatives, since mutation carriers are at an increased risk of developing CRC and endometrial cancer (up to 70%) as well as other cancers, and a close surveillance has been observed to improve morbidity and mortality1-3. Sporadic MSI-H cases can also be discriminated from HNPCC by detecting the B-RAF V600E mutation which is highly associated with the presence of MLH1 promoter methylation. Furthermore, MSI has been validated in independent and prospective studies as a good prognosis marker and has been reported to have an independent impact on the outcome of stage II CRC patients4-6. Other studies have also observed an absence of benefit from 5-fluorouracil-based adjuvant chemotherapy7-10 which adds up to the importance of assessing MSI status prior to therapy decisions.
MSI testing, using panels of microsatellites or looking at the loss of MMR proteins by immunohistochemistry (IHC), is now recommended on the Spanish Society of Pathology (SEAP) and the Spanish Society of Medical Oncology (SEOM) guidelines for biomarker testing in localized CRC due to its strong predictive value for deciding on adjuvant treatment and as a first step in the identification of putative hereditary cases11. Despite its clinical relevance MSI screening has not been widely implemented12.
MSI-H tumors have also been described to display common clinicopathological characteristics such as proximal location, solid growth pattern, mucinous differentiation, presence of Crohn-like lymphoid reactions and tumor-infiltrating lymphocytes, which has allowed the publication of several MSI prediction models based on pathological parameters, mostly pointing at identifying Lynch syndrome patients13-16. The aim of this study was to further validate with a prospective series, two previously published logistic models for the prediction of MSI-H, RERtest6 and RERtest8, based on clinicopathological features and developed as a result of the optimization of an initial model with a multicentric validation17,18.
MATERIALS AND METHODS
Patient characteristics and tissues
A final series of 206 prospective primary colorectal tumor samples surgically resected from 199 patients were evaluated, and the clinicopathological variables included in the RERtest6 and RERtest8 models were recorded as previously described17,18. Briefly such features included tumor location, proximal or distal to the splenic flexure (including rectum), growth pattern, expansive or infiltrative, presence of peritumoral Crohn-like lymphoid reactivity which was considered positive when at least three nodular aggregates of lymphocytes were present within a single low power field (4x), percentage of solid and mucinous differentiation and presence of tumor infiltrating lymphocytes (TIL) characterized by the finding of at least four intraepithelial lymphocytes in a high power field (40x) (figure 1).
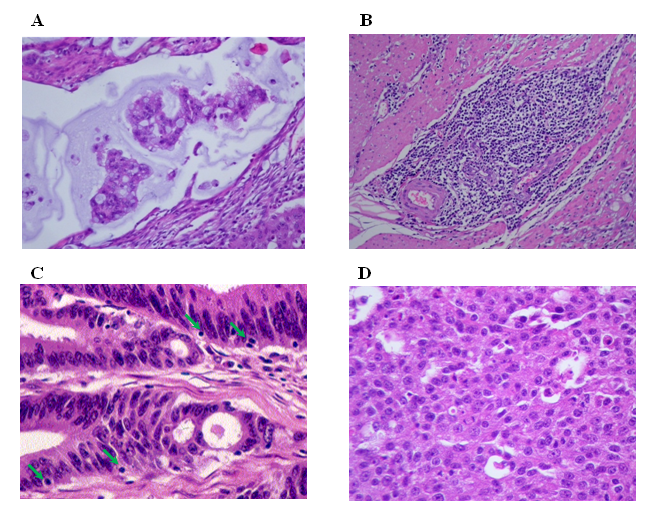
Figure 1: Histological parameters included in the RERtest6 model. A: Extracellular mucinous differentiation (H&E, 400x), B: “Crohn like” inflammatory response (H&E, 200x), C: Tumor infiltrating lymphocytes (TIL) (H&E, 400x), D: Solid pattern (H&E, 200x).
The RERtest8 also includes expression of Ki-67 and p53 assessed by immunohistochemistry. Loss of MMR proteins MLH1, MSH2 and MSH6 could be evaluated in 197 cases. Clinicopathological features of the MSI stable (MSS) and MSI-H cohorts are shown in table 1.
Table 1: Clinicopathologic features of the MSS and MSI-H cohorts. P-values were determined using Fisher’s exact test for categorical variables and Wilcoxon test for numerical variables. P-values less than 0.05 were considered statistically significant.
| Categorical variables | MSS (%), n=185 | MSI-H (%), n=21 | P-value |
| Age | 71 ± 10.7 | 70 ± 12.2 | 0.774 |
| Location | |||
| Proximal | 70 (37.8) | 20 (95.2) | <0.001 |
| Distal | 115 (62.2) | 1 (4.8) | |
| Growth pattern | |||
| Infiltrative | 127 (68.6) | 12 (57.1) | 0.328 |
| Expansive | 58 (31.4) | 9 (42.9) | |
| Crohn-like | |||
| Present | 52 (28.1) | 11 (52.4) | 0.042 |
| Absent | 133 (71.9) | 10 (47.6) | |
| Tumor-infiltrating lymphocytes | |||
| Present | 28 (15.1) | 7 (33.3) | 0.059 |
| Absent | 157 (84.9) | 14 (66.7) | |
| Numerical variables | MSS (mean ± SD) | MSI-H (mean ± SD) | P-value |
| Solid pattern (%) | 16.9 ± 20.8 | 25.5 ± 30.3 | 0.186 |
| Mucinous pattern (%) | 6.8 ± 18.8 | 16.4 ± 20.8 | <0.001 |
| Ki67 proliferative index (%) | 61.6 ± 21.0 | 67.4 ± 17.3 | 0.233 |
| p53 overexpression (%) | 40.0 ± 35.6 | 11.2 ± 16.0 | 0.004 |
Prediction of the MSI status
RERtest6 and RERtest8 models were applied to all cases prior to the assessment of MSI-status. Cases presenting a probability value of being MSS lower than 80% (P<0.8) were assigned as predicted MSI-H. An example of a MSI-H case predicted by the two models is illustrated in figure 2.

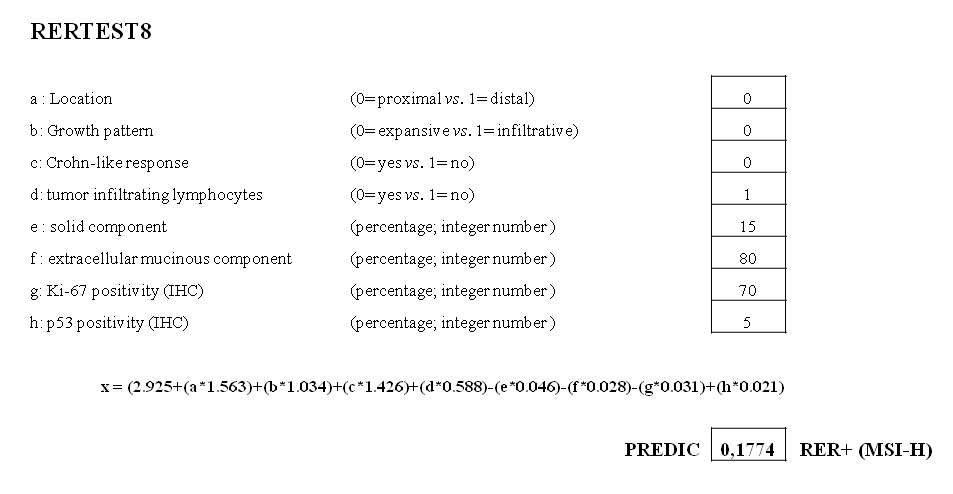
Figure 2: Example of a MSI-H case predicted using RERtest6 and RERtest8. Both optimized models are freely available, in an easily handled Microsoft’s Excel format, on our website (www.biopat.es). As shown, either 0 or 1 should be included in cells corresponding to cathegorical variables (a-d), the percentage of solid or mucinous component in cells e and f, and the percentage of Ki-67 or p53 IHC positive tumor cells in cells g and h, when using RERtest8. Cases exhibiting a probability value of being MSS lower than 0.8 are classified as MSI-H.
DNA extraction
Genomic DNA was extracted from ten 5-µm-thick sections of paired normal and tumor samples by macrodissection of selected areas followed by proteinase digestion and purification using a QIAamp DNA FFPE Tissue Kit. DNA quality was assessed by amplification of a 268 bp fragment of the β-globin gene.
Microsatellite instability analysis
Appropriate amounts of paired normal and tumor DNA, depending on the panel used, were employed to assess the MSI status. The first 145 cases in our series were evaluated using the consensus NCI panel composed by the five microsatellites (BAT25, BAT26, D5S346, D2S123 and D17S250) in a multiplex PCR19. The Promega MSI Analysis System containing five mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24 and MONO-27) for MSI evaluation and two pentanucleotide repeat markers (Penta C and Penta D) for quality assurance was used to assess the latter 61 cases. Fluorescent amplicons from both PCR systems were analyzed on an automated ABI PRISMÒ 310 Genetic Analyzer using the GeneScan software (PE Applied Biosystems).
According to the consensus definitions of the US NCI, tumors were classified as exhibiting high microsatellite instability (MSI-H) when 30% or more of the tested loci resulted unstable and non-MSI-H when they were less than 30%. Tumors exhibiting low microsatellite instability (1 unstable marker out of 5) were considered together with stable tumors.
B-RAF mutation analysis
The presence of the V600E B-RAF mutation was determined in all MSI-H tumors in order to discriminate sporadic cases. The first 10 cases were assessed by direct Sanger sequencing using the ABI PRISM® BigDye Terminator kit v1.1. The latter 11 cases were studied by selective PCR amplification of the mutant allele followed by hybridization on a strip with mutant specific probes (Stripassay, Viennalabs).
Immunohistochemical analysis
All IHC analyses were performed following an avidin–biotin immunoperoxidase procedure. IHC was performed on 4-mm-thick paraffin sections after rehydration. Endogenous peroxidase activity was inhibited with 0.1% hydrogen peroxide; deparaffinized sections were pretreated to induce epitope retrieval and were independently incubated with their corresponding monoclonal antibody. Secondary reagents were biotinylated horse anti-mouse antibodies (Vector Laboratories, Burlingame, CA, USA) and avidin–biotin complexes (Vectastain ABC Elite, PK6100, Vector Laboratories). A 3,30-diaminobenzidine was used as the final chromogen and Harris-modified hematoxylin as the nuclear counterstain. Positive and negative controls, in which the primary antibody was omitted, were included in each experiment.
Mouse monoclonal antibodies clone DO-7 and clone MIB-1 (DakoCytomation, Denmark A/S) were used to detect p53 and Ki-67 proteins, respectively. IHC evaluation was conducted double-blind by scoring the estimated percentage of tumor cells showing nuclear staining.
MMR gene expression was assessed using the following primary monoclonal antibodies: anti-hMLH1 (clone G168-15), anti-hMSH2 (clone G219-1129) and anti-hMSH6 (clone 44) (BD Biosciences Pharmingen). The absence of nuclear staining observed in the tumor cells together with a positive internal control (lymphocytes within tumor, normal mucosa or stromal cells, endothelial cells) was considered as negative. Specimens with positive staining in a small cluster of neoplastic cells were considered to express the proteins20.
RESULTS
Microsatellite instability was detected in 21 (10.1%) cases in the series, loss of MMR proteins could be demonstrated in 15 of these cases; three cases could not be evaluated and the remaining three showed conserved protein expression. There were no cases presenting MMR deficiency and a MSS or MSI-L phenotype, that could be considered MSI false negative by neither of the two MSI assessment panels employed, although 9 cases that had not rendered evaluable results with the NCI panel could be recovered using the Promega MSI kit (data not shown).
The B-RAF V600E mutation was detected in 12 out of these 21 MSI-H cases, which would indicate an incidence of 57% of sporadic MSI-H cases in our series.
As expected, MSI-H cases were significantly of proximal location, presented Crohn-like lymphoid reactivity, and had higher percentage of mucinous differentiation and lower p53 overexpression. The presence of expansive growth pattern, tumor infiltrating lymphocytes, solid differentiation and a higher proliferative index, although more common in the MSI-H cohort, didn’t reach statistical significance in this series. No age difference could be observed between MSS and MSI-H cohorts (table 1).
Both our models, RERtest6 and RERtest8, maintained robustness and were able to predict MSI-H with a negative predictive value of 94.4% and 95.0% respectively. This result allows the reduction of cases to be tested to only the 10% of cases predicted as MSI-H that should be confirmed. The performances as well as the statistical parameters achieved by both our models are shown in tables 2 and 3. The different methodology employed for MSI-H testing had no influence on the results of neither of the models.
Table 2: Performance and statistical parameters obtained with the RERtest6 model.
| MSS / MSI-L | MSI-H | ||
| RERtest6 MSI-H | 18 | 11 | 29 |
| RERtest6 MSS/MSI-L | 167 | 10 | 177 |
| 185 | 21 | 206 | |
| Accuracy | 86.41% | ||
| Sensitivity | 52.38% | ||
| Specificity | 90.27% | ||
| Positive predictive value | 37.93% | ||
| Negative predictive value | 94.35% |
Table 3: Performance and statistical parameters obtained with the RERtest8 model.
| MSS / MSI-L |
MSI-H |
||
| RERtest8 MSI-H | 13 | 12 | 25 |
| RERtest8 MSS/MSI-L | 172 | 9 | 181 |
| 185 | 21 | 206 | |
| Accuracy | 89.32% | ||
| Sensitivity | 57.14% | ||
| Specificity | 92.97% | ||
| Positive predictive value | 48.00% | ||
| Negative predictive value | 95.03% |
DISCUSSION
The strong prognostic value attributed to MSI-H, both in sporadic and HNPCC cases and its association with a poor response to 5-fluorouracil-based regimes has emphasized the need to assess MSI status in all colorectal cancer cases independently from whether they meet criteria for HNPCC. Despite such evidences, several studies have demonstrated the deficient implementation of MSI testing in general practice, especially in older patients, which strengthens the need for screening tools to make MSI-H testing more feasible.
The aim of this study was to validate two MSI-H prediction models, RERtest6 and RERtest8, based on clinicopatological features, and obtained previously as a result of the optimization of an initial model17,18. This optimization first involved the multicentric validation of the original prediction model with a series of 265 unselected primary colorectal carcinomas prospectively collected from five different centers. Once the original model had been proven consistent enough to overcome interobserver variability, another 350 cases collected in our institution were also included in a statistical analysis to consolidate the parameters that gave rise to the RERtest models (figure 1).
In this prospective validation series several parameters typically associated with an MSI-H phenotype, such as expansive growth pattern, solid differentiation and presence of tumor infiltrating lymphocytes, although predominant in the MSI-H cohort, did not research statistical significance. This fact advocates in favor of using a multifactor model to predict MSI-H status rather than relying on the presence of characteristic features alone.
Most published MSI-H prediction models, with the exception of MMR index21-22, are focused on identifying HNPCC patients. In contrast, both our initial model and the optimized models RERtest6 and RERtest8, were constructed to be applied to an unselected population and to allow the identification not only of those patients at risk of presenting HNPCC, but also of all those other patients that present a better prognosis, that in our series represent a 57% of all MSI-H cases.
In our series 9 (43%) of our MSI-H cases did not harbor the V600E B-RAF mutation, which correlates with a higher probability of presenting Lynch syndrome, furthermore 6 of these patients were aged over 60, including an 82 years old patient. Several studies and guidelines now recommend systematic testing of all CRC patients by means of MSI or MMR IHC6 which supports the use of non-age restricted prediction models.
The main feature of the RERtest models, when compared to other published models13-16, 21-22, is that they have been developed with the objective of reducing the number of cases to be tested for MSI; rather than predicting MSI-H per se; consequently achieving a high negative predictive value has been the main priority even over sensitivity. In this prospective validation both our models were able to achieve a negative value, close to 95%, that would reduce the need for MSI testing to just the 10% of cases predicted as MSI-H.
The straightforward evaluation of the parameters included in the RERtest6 model, already part of a standard pathological examination, and the fact that the model is freely available on our website (figure 1), renders it a very useful tool to be incorporated into routine practice. Also, the RERtest8 model may be a good option in those laboratories already performing Ki67 and P53 IHC assays regularly on their CRC cases, since it achieves slightly better statistical parameters.
Independently of the model used, we propose confirming the MSI-H phenotype of all predicted cases by MSI PCR, due to its reported superior sensitivity11,23, or in its defect by MMR IHC. The use of both methods, MSI PCR and MMR IHC, whenever possible, would of course reduce the risk of a false negative to a minimum. Molecular testing of the small proportion of MSI-H predicted cases is a feasible approach that ensures the correct identification of patients with good prognosis or at risk for HNPCC and can contribute to therapy and clinical management decisions.
ACKNOWLEDGMENT
The authors thank Eva Torija from BIOPAT for her secretarial assistance in data collection.
REFERENCES
- Funkhouser W., Lubin I, Monzon F., Zehnbauer B., Evans J., Ogino S., Nowak J. Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas. A report of the association for molecular pathology. J Mol Diagn. 2012; 14: 91-103.
- Vasen H., Blanco I., Aktan-Collan K., Gopie J., Alonso A., Aretz S, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013; 62: 812-823.
- Ward R., Hicks S. and Hawkins J. Population-based molecular screening for Lynchsyndrome: implications for personalized medicine. J Clin Oncol. 2013; 31: 2554-62.
- Merok M., Ahlquist T., Røyrvik E., Tufteland K., Hektoen M., Sjo O., et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Annals of Oncology 2013; 24: 1274-1282.
- Mouradov D., Domingo E., Gibbs P., Jorissen R., Li S., Soo P., et al. Survival in stage II/III colorectal cancer is independently predicted by chromosomal and microsatellite instability, but not by specific driver mutations. Am J Gastroenterol. 2013; 108: 1785-1793.
- Sagaert X. Prognostic biomarkers in colorectal cancer: where do we stand?. Virchows Arch. 2014; 464: 379-391.
- Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003; 349: 247-57.
- Sargent DJ, Marsoni S, Thibodeau SN, Labianca R, Hamilton SR, V. Torri V., et al. Confirmation of deficient mismatch repair (dMMR) as a predictive marker for lack of benefit from 5-FU based chemotherapy in stage II and III colon cancer (CC): a pooled molecular reanalysis of randomized chemotherapy trials. J Clin Oncol 26. 2008; No 15S: 4008.
- Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective Mismatch Repair As a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J Clin Oncol. 2010; 28: 3219-3226.
- Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, et al. DNA Mismatch Repair Status and Colon Cancer Recurrence and Survival in Clinical Trials of 5-Fluorouracil-Based Adjuvant Therapy. J Natl Cancer Inst. 2011; 103: 863-875.
- García-Alfonso P., Salazar R., García-Foncillas J., Musulén E., García-Carbonero R., Payá A., et al. Guidelines for biomarker testing in colorectal carcinoma (CRC): a national consensus of the Spanish Society of Pathology (SEAP) and the Spanish Society of Medical Oncology (SEOM). Clin Transl Oncol. 2012; 14: 726-39.
- Beamer L., Grant M., Espenschied C., Blazer K., Hampel H., Weitzel J., et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012; 30: 1058-63.
- Hyde A., Fontaine D., Stuckless S., Green R., Pollett A., Simms M., et al. A histology-based model for predicting microsatellite instability in colorectal cancers. Am J Surg Pathol. 2010; 34: 1820-1829.
- Jenkins MA, Hayashi S, O’Shea, Burgart LJ, Smyrk TC, Shimizu D, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology 2007; 133:48–56.
- Greenson JK, Huang S, Herron C, Moreno V, Bonner JD, Tomsho LP, et al. Pathologic predictors of mirosatellite instability in colorectal cancer. Am J Surg Pathol. 2009; 33:126–133.
- Jass JR. HNPCC and sporadic MSI-H colorectal cancer: a review of the morphological similarities and differences. Familial Cancer 2004; 3: 93-100, s.l.
- Colomer A., Erill N., Vidal A., Calvo M., Roman R., Verdú M., et al. A novel logistic model based on clinicopathological features predicts microsatellite instability in colorectal carcinomas. Diagn Mol Pathol. 2005; 14: 213-223.
- Román R., Verdú M., Calvo M., Vidal A., Sanjuan X., Jimeno M., et al. Microsatellite instability of the colorectal carcinoma can be predicted in the conventional pathologic examination. A prospective multicentric study and the statistical analysis of 615 cases consolidate our previously proposed logistic regression model. Virchows Arch. 2010; 456: 533-541.
- Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998; 58: 5248–5257.
- Valentini AM, Armentano R, Pirrelli M, Gentile M, Caruso ML. Immunohistochemical mismatch repair proteins expression in colorectal cancer. Appl Immunohistochem Mol Morphol. 2006; 14: 42–45.
- Joost P., Bendahl P., Halvarsson B., Rambech E. and Nilbert M. Efficient and reproducible identification of mismatch repair deficient colon cancer: validation of the MMR index and comparison with other predictive models. BMC Clinical Pathology 2013; 13: 33-40.
- Halvansson B., Anderson H, Domanska K, Lindmark G and Nilbert M. Clinicopathologic factors identify sporadic mismatch repair-defective colon cancers. Am J Clin Pathol. 2008; 129: 238-244.
- Cicek M., Lindor N., Gallinger S., Bapat B., Hopper J., Jenkins M., et al. Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors. Results from the Colon Cancer Family Registry. J Mol Diagn. 2011; 13: 271-81.